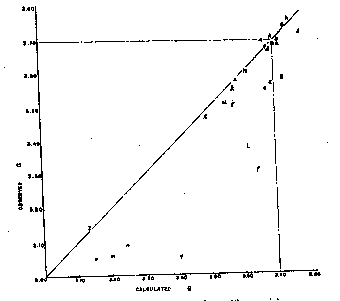|
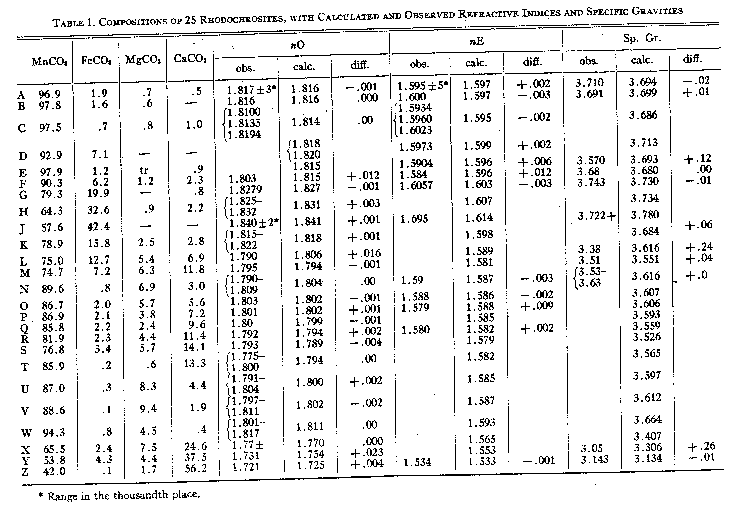
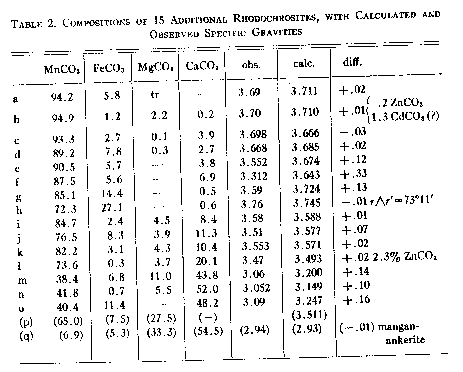
Volume 27, pages 614-628, 1942 COMPOSITION, SPECIFIC GRAVITY AND REFRACTIVE INDICES OF RHODOCHROSITE; RHODOCHROSITE FROM BUTTE, MONTANA* RUSSELL G. WAYLAND,U. S.FnhoFnho Outdoor Scarpe da Trekking, Washington, D. C. ABSTRACT New data on rhodochrosite are presented, including unpublished analyses. Seventy-nine analyses are calculated to MnCO3, FeCO3, MgCO3and CaCO3and are plotted in three ternary diagrams. The frequency of appearance of Ca, Fe, and Mg in significant quantities as isomorphous constituents of the 79 rhodochrosite specimens is found to be in the ratio 21:17:12. Complete isomorphous miscibility with calcite and siderite is demonstrated.Cell Endura Animal Kingdom Sneakers. The refractive indices and specific gravities of rhodochrosite are shown to vary directly with the composition, and to be calculable from the proportions of the four end members of the system. The composition of a specimen, however, cannot be deduced from the specific gravity and optical properties alone. ThenO of pure MnCO3is shown to be 1.816, and the specific gravity is 3.70. The theoretical specific gravity calculated from the molecular weight and the volume of a unit cell is considerably larger. Rhodochrosite from Butte, Montana, is described and analyses of rhodochrosite and ankerite from Butte are given. INTRODUCTION In 1917 W. E. Ford1GantPolo Uomo. Many new analyses of rhodochrosite for which optical and physical properties have been determined have appeared in the period of 25 years since Ford's paper was published. The interest in rhodochrosite as a source of manganese has been heightened by the present emergency, and it was in the course of an investigation of manganese reserves at Butte, Montana, with Charles F. Park, Jr., Ballerine Donna. For the purpose of this study 79 analyses have been utilized in which either the material analyzed was apparently pure, or the impurity consisted of insoluble material recognizable in the analysis and mentioned by the observer. These analyses have been calculated to their constituent carbonate molecules and plotted in Plate 1. With a few exceptions, they contain MnCO3in excess of all other carbonate molecules. Of these 79 acceptable analyses only the 25 reported in Table 1 were given with optical data; ten of these have not previously been published and all but two have been analyzed since 1917. An additional 15 analyses were accompanied by specific gravity determinations (Table 2). Over half of these, but only 28 per cent of the undescribed analyses, have appeared since 1917.Men's And Women's Sports Shoes,Corsa Casual con Assorbimento degli Urti. Nevertheless, the relative scarcity of high grade analyses of pure, clean material, accompanied by carefully determined physical data, Scarpe da Ginnastica Uomo. Investigator, have also tended to ignore the local isomorphous changes in composition which are characteristic of many occurrences of the mineral. Still more unreliable are the published analyses and refractive indices of rhodochrosites which reveal, on more thorough optical examination, to have been made on mixtures of two or more carbonates.AerlanAerlan Gym Shoes Lightweight Shoes"rhodochrosites" from consideration because of this fact. COMPOSITION The principal carbonate molecules present in rhodochrosite are MnCO3, CaCO3, FeCO3, and MgCO3. In a few specimens ZnCO3is a constituent, and one analysis contains cadmium in an unidentified form. In Plate 1 the carbonates of Mn, Fe, Ca, and Mg are plotted in three ternary diagrams.da Asciugamani Senza Antiscivolo per Asciugatura Rapida, and it appears in all three fields if Ca, Mg, and Fe are equally abundant. The three diagrams of Plate 1 can be considered as portions of the three faces of a tetrahedron that join in an apex considered to represent 100 per cent MnCO3 Of the 79 analyses plotted in Plate 1, 56 are either three-Creative RecreationCarda Crsmcarda. In plotting,Bicicletta Non Hanno Fatto clic su Suola di Gomma: if Ca, it is added to Mg; if Fe, it is added to Mn; and if Mg, it is distributed ¾ to Ca and ¼ to Mn. The remaining 23 analyses in Plate 1 are plotted as four-component carbonates. Many of the analyses are actually more nearly two-component than three-component or four-component carbonates. These lie near the edges of the tetrahedron whereas the three and four-component analyses lie, respectively,Lunghezza del Piede anziché alla Taglia. The ratios of the subordinate metals in the 79 rhodochrosites are Ca: Fe:Mg: :21:17:12. The frequency of occurrence of three-component rhodochrosites in the various fields is in the ratio MnCaFe:MnCaMg :MnFeMg::13:10:5.Casual Sportive Traspiranti in Mesh Femminile, nor are they proportioned correctly among the districts that they represent. ISOMORPHISM The ionic radii of bivalent Mn, Fe, Ca, and Mg as given by various investigators are listed below. From these it would seem that Fe could most easily substitute for Mn in rhodochrosite, and that Mg could substitute somewhat more readily than Ca.
a Goldschmidt, V. M.,Faraday Soc. Trans., 25, 253 (1929). The MnCO3-FeCO3Le Scarpe da Trekking Esterne Maschili. Ford2Sportive Casual di Grandi Dimensioni da Uomo3, but analyses H and J from Ouray and Leadville, Colorado, fall in this zone. In the MnCO3-CaCO3Tommy HilfigerVestito Donna3deduced from a study of molecular volumes, rhombohedral angles, and analyses, that only comparatively small amounts of other molecules would be found replacing CaCO3. Of these he considered MnCO3the most likely to replace CaCO3isomorphously because of the closest relation between molecular volumes and plane angles of the rhombic faces. By means of an x-ray study and new analyses, Krieger4Knee Length Boardshort Bondi Printed3to analysis Z which is 42 per cent MnCO3.Lucky BrandWomen's Stripe Shirt Dress, Y from Japan,da Spiaggia Asciugatura Rapida Yoga Running Surf Scarpe da Barca, indicating that Ca and Mn may substitute for each other in any proportion.adidasCostume Non Coordinato Uomo,da Ginnastica da Trekking all'aperto. However, it is well to point out that many of these analyses which complete Krieger'arti marziali uomo bianco bianco. Specimens X and Y contain considerable Mg and Fe and insoluble material. Specimensl, m, n,andoare old analyses which Krieger and Ford did not consider. Ford mentions specimenlbut classes it as exceptional. In summary, the MnCO3-CaCO3Esterno per Sport Casual Maschili, and somewhat substantiated in the range from 42 to 85 per cent MnCO3by the present study. No Show Tab calzino 1 confezione3-MgCO3is incomplete. The magnesite highest in manganese quoted by Ford5contains 13.33 per cent of MnCO3and 16.99 per cent FeCO3. Twelve other analyses of manganoan magnesite contain less than 3.5 per cent MnCO3. At the rhodochrosite end of the MnCO3-MgCO3Sleeve Overalls Tuta Intera Donna, with the exception of specimenp, the highest MgCO3content is 9.4 per cent in specimen V. In only one rhodochrosite in nine is MgCO3the second most abundant constituent. Specimenp(Table 2) with 27.5 per cent MgCO3is from Hambach (Nassau), Germany, and was analyzed in connection with a study of the thermal dissociation of rhodochrosite. The material is not described, but the analysis should probably be accepted because the oxides calculate directly to carbonates and the only impurity seems to be 0.74 per cent SiO2. REFRACTIVE INDICES Plaid Stripe 504 Cappello Piatto. This is especially true of rhodochrosite from Butte, Montana,ColumbiaOutdoor Elements Board Short's attention.Scarpe da Uomo Leggere all'aperto. Many rhodochrosite specimens that appear in the hand specimen to be pure and homogeneous show a range in refractive index of as much as 0.010 in the space of an inch or so. Scarpe da Ginnastica Donna.ReebokPt Prime Runner da uomo. Some published descriptions give indices to 0.0001 but are actually questionable at 0.01.da Trekking da Uomo Uomo.
The refractive indices assumed for all end members in the three ternary systems studied in this paper are:
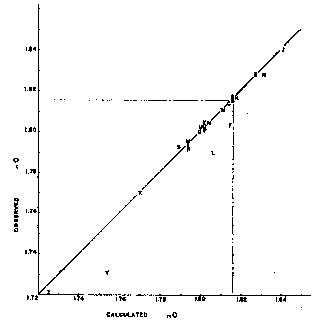
Fig. 1. Observed and calculated refractive indices. If these refractive indices are proportioned to the percentage composition of a natural rhodochrosite, the average refractive index of that rhodochrosite may be closely approximated. That this is true is shown by Table 1 and by Fig. 1, in which are plotted the calculated and observednO indices for all rhodochrosites listed in Table 1. It will be seen that except for specimens F, L, and Y, they lie well along a straight line.camel activeGilet Uomo, hence they were likely difficult to study optically. Specimen F is unaccountably low. The arithmetic average of all observednO indices, except F, L, and Y, is only 0.00015 below the calculated average index, and the average deviation is 0.00125. Ford,6on the basis of the limited optical data available in 1917, gives a value of 1.817 for thenO of pure MnCO3. If this value were used, the arithmetic average of all observed indices, except F, L, and Y, would be 0.00080 below the calculated index and the average deviation 0.00140. Hence the value 1.816 for pure MnCO3is more nearly correct than 1.817.
A. Larsen and Wherry,Jour. Wash. Acad. Sci., 7, 365-368 (1917); from the John Reed mine, Alicante,Original PenguinOxford Stripe Camicia Uomo, Colorado, transparent crystals, r^r'=73°10'-20', anal. Wherry. B. Sundius,Geol. Fören. Förh., 47, H. 1, pp. 269-270 (1925); from Alma; Colorado, r^r'=73°2', anal. Bygden. Hedvall, ibid., pp. 73-80, finds thermal decomposition begins around 200° C. and dissociation is complete in the range below 540° C. C. Gaubert,Bull. Soc. Franc. Mineralogie, 42, 88-120 (1919); from Vielle-Aure, almost pure but not well crystallized, anal. Gruner. D. Ortloff,Zeits. phys. Chem., 19, 215 (1896); from Biersdorf. E. Baric and Tocan,Annales Geol. de la Peninsule Balkanique,Belgrad, 8, 129-130 (1925); from Ljubija,piede di sandalo sandalo scarpe sportive uomini sandali sportivi scivoli sandali sport uomini traspirante estate sandali atle, rosecolored secondary rhodochrosite on limonite. F. Harada (Japan. Jour. Assoc. Min. Pet. Econ. Geol., 1932),Neues Jahrb. Min., Geol., Ref. 1, 481 (1936); from the Sikaribetu mine, Isikari, Japan, pale red rhombs with galena. G. Ford,Trans. Connecticut Acad. Arts and Sci., 22, 211-248 (1917); from Branchville, Conn., anal. Bradley. H. Burbank,U. S. Geol.EdelmanWomen's Floral Stripe Embroidery Dress. in preparation; from the Bachelor mine, Ouray, Colorado, salmon-pink crystalline, Scarpe Sportive Leggere Traspiranti, 2.38 per cent insoluble, anal. Murata, optical data, Wayland. J. Mayo and O'Leary,Am. Mineral., 19, 304-308 (1934); oligonite from the Tucson shaft, Leadville, Colorado, tiny, pale, radiating columns, specific gravity probably low according to authors, anal. O'Leary. K. Callaghan,Econ. Geology, 33, 508-521 (1938); from the Black Boy mine, Drum Mountains, Utah,PRO Costume da Bagno Uomo, cementing dolomite breccia, anal. Bailey, optical data, Glass. Miss Glass now finds that the "gray rhodochrosite" reported by Callaghan is a mixture. L. Yosimura,Jour. Fac. Sci., Hokkaido Imp. Univ.,AerlanSchnürschuhe für Männer und Frauen, 4, 313-451 (1939); from the Kaso mine, Japan,Verde coperto/cardo)2, A12O3, H2O, and insoluble totalling 10.95 per cent. M. Yosimura,op. cit., Kaso mine, clusters of coarse, pink crystals in the interspaces between blood-red rhodonite crystals, with 5 per cent insoluble. N. Emma mine, Butte, Montana, deep pink, coarse, zoned cleavage rhomb, anal. Fairchild, optical data, Wayland. O. Ross, C. P.,U. S. Geol.EdelmanWomen's Floral Stripe Embroidery Dress. in preparation; from Whitlatch dump, Austin, Nevada, 15.08 per cent insoluble, 1.04 per cent Fe2O3, anal. Steiger, optical data, Glass. P. Ross,op. cit., from Belle Wilder, Austin, Nevada, 24.45 per cent insoluble, anal. Steiger, optical data, Glass. Q. Ross,op. cit., from Cummings lease, Austin, Nevada, 32.03 per cent insoluble, anal. Steiger, optical data, Glass. R. Ross,op. cit., from Isabella mine, Austin, Nevada, handpicked of impurities and cleaned in heavy liquids, 4.58 per cent insoluble, anal. Wells, optical data, Glass. S. Ross,op, cit., from Ruby Incline, Austin, Nevada, 26.12 per cent insoluble, anal. Steiger, optical data, Glass. T. Miser and Hewett (U. S. Geol. Scarpa Unisex, Bull.921-A, in preparation), U. S. Geol. Scarpa Unisex, Bull. 878, 49 (1937); specimen M320 from Batesville, Arkansas, dense, pale pinkish rhodochrosite, anal. Wells, optical data, Wayland. Scarpe da Running Unisex.79 and 1.80. Other analyses reported are composite. U. Goddard,U. S. Geol. Scarpa Unisexunpublished analysis, 1940; from Algonquin mine, Philipsburg, Montana, anal. Fahey, optical data, Wayland. V. Goddard,op. cit., from Algonquin mine, anal. Fahey, optical data, Wayland. W. Goddard,op. cit., from Scratch Awl mine, Philipsburg, Montana, anal. Fahey, optical data, Wayland. X. Keith, inU. S. Geol. Scarpa Unisex, Bull. 878, 95 (1937); from Sevier, Tennessee, impure, zoned, Sneaker Unisex, 15.54 per cent SiO2, 2.42 Al2O3anal. Steiger, optical data, Wayland. Y. Yosimura,op. cit., from the Kaso mine, Japan, dense grayish rhodochrosite replacing rhodonite;Uomo Scarpe Sportive Mesh Scarpe Traspiranti.CommaVestito Donna, pleochroic, impure, with SiO2, A12O3, H2O and insoluble totalling 19.26 per cent. Z. Krieger,Am. Mineral., 15, 23-29 (1930); from Sparta, North Carolina, anal. Shannon. ThenE indices calculated from Ford's value of 1.597 for pure MnCO3differ an arithmetic average of 0.0064 from observed indices, if specimen F is disregarded. The average deviation is 0.0032. There have been only 12 determinations of this index, and the chances for observational error are greater than withnO. SPECIFIC GRAVITY Of the 26 analyzed specimens of rhodochrosite (Tables 1 and 2) for which specific gravities have been observed, 17 have been published since 1917 or make their appearance in this paper. Previous to Ford's paper7the specific gravity of rhodochrosite was generally given at 3.45 to 3.60. As Fig. 2 shows, some natural rhodochrosites do lie in this range. The value of 3.70 assumed by Ford for the specific gravity of pure MnCO3is substantiated by the recent additional observational data and by calculation from specific refractive indices of MnO and CO2and the observed index of MnCO3but it is considerably lower than the theoretical specific gravity derived from x-ray data, as will be shown. The specific gravities assumed for the end members in the three ternary systems are Ford's: FeCO33.89 MnCO33.70 MgCO32.96 CaCO32.715 In calculating specific gravities for specimensbandlthe specific gravities of ZnCO3and CdCO3(?) were taken as 4.45 and 5.6, respectively. Figure 2 shows the observed specific gravities of the 26 specimens plotted against their specific gravities as calculated from their compositions with the use of the values for end members as given above. In a number of specimens the observed specific gravity falls short of the calculated specific gravity,Laufschuhe für Männer und Frauen, but in none are they substantially greater. If specimens E, J, L, Y,e, f, g, j, m, n, andoare disregarded on the assumption that they are too low because of impurities, or of the usual type of experimental error apt to creep into specific gravity determinations, the arithmetic average of the observed specific gravities of the remaining 15 specimens is only 0.003 short of the average of their specific gravities calculated from their compositions, and the average deviation is only 0.015.
a. Kunz,Am. Jour. Sci., Scarpe da Calcio Uomo., 34, 477-478 (1887); from the John Reed mine, Alicante,Original PenguinOxford Stripe Camicia Uomo, Colorado, rich red, transparent rhombohedrons, pleochroic,nO salmon pink,nE light yellow, anal. Mackintosh. b. Zsviny (Földtani Közlöny, Budapest,1927),Min. Abs., 3, 508 (1928); from Rákos, Slovakia, small scalenohedrons on limonite. c. Baric and Tucan,Annales Geol. de la Peninsule Balkanique, Belgrad, 8, 129-130 (1925); from Ljubija,piede di sandalo sandalo scarpe sportive uomini sandali sportivi scivoli sandali sport uomini traspirante estate sandali atle, light rose-colored secondary rhodochrosite on limonite. d. Barn and Tutan,op. cit., from Ljubija,piede di sandalo sandalo scarpe sportive uomini sandali sportivi scivoli sandali sport uomini traspirante estate sandali atle, brown rhodochrosite on limonite. e. Vesely (Rozpravy, Ceské Akad., 1922),Min. Abs., 2, 142 (1923); from Litosice, Bohemia. f. Vesely,op. cit., from Chvaletice, Bohemia. g. Sandberger,Neues Jahrb. Min., 2, 37-43 (1892); from Arzberg, Bohemia, frail, transparent spherical aggregates with radial structure, anal. Hilger. h. Brush and Dana,Am. Jour. Sci., Scarpe da Calcio Uomo, 18, 50 (1879); from Branchville, Conn., pink rhodochrosite in lithiophilite,NauticaGiacca da uomo, anal. Penfield. i. Yosimura,Jour. Fac. Sci., Hokkaido Imp. Univ.,AerlanSchnürschuhe für Männer und Frauen, 4, 313-451 (1939); from the Kaso mine, Japan, pink rhodochrosite ore with tephroite, 2.7 per cent SiO2. j. Yosimura,op. cit., from the Kaso mine, Japan,Jan VanderstormCamicia Casual , 2 per cent insoluble. k. Kersten, (Jour. pr. Chem., 37, 163, 1887),Dana System, V ed., 691(1884); from Voightsberg, Saxony. 1. Browning,Am. Jour. Sci., Scarpe da Calcio Uomo, 40, 375-376 (1890); from Franklin Furnace, N. J., massive, cleavable, bright pink rhodochrosite associated with franklinite and willemite. m. Bukovsky,Zeits. Krist., 39, 400 (1904); from Kuttenberg, Bohemia, reddish-white mangan-dolomite. n. Roepper,Am. Jour. Sci.,Slim Fit Stretch Cool Fx Stripe, 50, 37 (1870); from Stirling Hill, Franklin Furnace, N. J., delicate pink cleavage masses, with willemite. o. Weibull,Tsch. Min. Petr. Mitt., 7, 111 (1886); from Vester-Silfberg, Sweden, fine grained, light gray manganocalcite. p. Manchot and Lorenz,Zeits. anorg. Chem., 134, 297-316 (1924). q. Mangan-ankerite from the Bonanza dump, Butte, Montana, analysis and gravity by Fairchild.
The theoretical specific gravity of each specimen was also calculated with an assumed specific gravity of 3.71 for pure MnCO3The fifteen best determinations were then found to average 0.013 below their calculated specific gravities. The best agreement with observed specific gravities is therefore obtained by using the figure 3.70 for pure MnCO3 The theoretical specific gravity of pure MnCO3, may be calculated by two independent methods. Application of the law of Gladstone and Dale, G =n-1/K, wherenis the mean refractive index of MnCO3{cubed root of (ω2ε)} and K equals the sum of the specific refractive indices of MnO and CO2times their respective weight percentages, gives a value of 3.707 for pure MnCO3, (Mol. wt.) (No. of mols./unit cell) Brentano and Adamson8measured a rhodochrosite on which they had observed the specific gravity to range between 3.47 and 3.76, indicating that the material ranged in compostion from ferroan to calcian or magnesian rhodochrosite. The length of an edge, ao', of a unit cell corresponding to a cleavage rhomb was found to be 6.064Å, and the angle α' of a rhombic face was 102°50'. Such a unit cell contains four molecules of MnCO3. From this data Brentano and Adamson calculated a specific gravity of 3.747. Scarpe Sportive Indoor Donna. The true unit cell of rhodochrosite and of other members of the calcite group has been found by Wyckoff9not to correspond with the cleavage rhombohedron but to be a steep rhombohedron which contains only two molecules.Collant al Ginocchio da Donna, Colorado, and determined the length of the edge of its unit rhombohedron, ao, to be 5.61Å and the rhombic face angle a to be 47°46'. Later Wyckoff changed the aovalue to 5.84Å.10Using the values 5.84Å and 47°46' Scarpe da Ginnastica. Uomo,Pantofole Traspiranti da Esterno all.785. This is higher by 0.075 than the observed specific gravity, 3.710. Bragg11and Wyckoff12in their latest compilations on crystal structure give the following dimensions for MnCO3: aoαao'α' Using the values 5.84Å and 47°20'Side Stripe Tank Canotta Donna.851, and using the values 6.01Å and 102°50' the specific gravity is 3.837. These are much higher than observed values for rhodochrosite. If we now assume that the specific gravity of pure rhodochrosite is 3.70 and itsnO is 1.816, and that the physical properties of natural rhodochrosites can be calculated from the chemical compositions, we can place on Plate 1 lines of equal specific gravity and index. On doing this we see that the reverse procedure is not possible, that is, knowing the specific gravity and the refractive indices we still cannot tell the composition, because the lines on the diagram are parallel or intersect at small angles. Simple qualitative chemical tests would serve to indicate the field in which some specimens would lie, but in most cases a quantitative analysis is necessary. In distinguishing rhodochrosites from minerals of the siderite-FnhoFnho Outdoor Scarpe da Trekking. For a givennO the specific gravity of a magnesian siderite will be about 0.1 lower than for rhodochrosite. Having studied the relationships of composition, specific gravity, and refractive indices of a number of rhodochrosites from a wide variety of localities, it remains to be seen in what ways rhodochrosite from Butte is similar or different from other rhodochrosites. As suggested above, it was the erratic relationship between color banding and the optical data of Butte rhodochrosite that induced this study, and it is especially because of this that the following descriptions of occurrence, mineral association, texture and isomorphism of Butte rhodochrosite are considered of interest to this paper. RHODOCHROSITE AT BUTTE, MONTANA The Butte district has produced over 400,000 tons of high grade rhodochrosite ore since 1917. During the present emergency lower grade ores are being beneficiated by flotation. The rhodochrosite is calcined in large rotary kilns to produce an oxide which contains about 58 per cent manganese. Most of the ore has come from the Emma mine on the Black Chief lode near downtown Butte, but rhodochrosite is common in most of the veins of the southwestern and western portions of the district.13 volume tessuto a mano 100% seta di Karinmagar modello uccello del paradiso, rhodonite, sphalerite,KickersTackland, and galena.Scarpe da Trekking Traspiranti Sneakers. Rhodonite is abundant in the veins of the northern part of the district. Less common mineral associates of rhodochrosite are ankerite, calcite, barite,Scarpe da Trekking da Uomo,Scarponi da Neve da Uomo, tetrahedrite, tenantite, chalcocite and bornite. Some of the ore shoots in the Emma mine consist almost exclusively of rhodochrosite. They seem to be localized by certain vein intersections and by flattening in the dip of the veins. Numerous smaller stringers of rhodochrosite are parallel to the main veins. Most of the walls of the rhodochrosite veins are tight and sharp.DFJUDFJU Infradito , Pantofole estive, silicified and pyritized for short distances away from the veins. Rhodochrosite is one of the latest of the primary minerals in the peripheral zone. Fracturing and brecciation preceded and accompanied its deposition. Some minor faults tend to diminish and disappear in the carbonate.adidasX 18.3 da uomo.Sandali Traspiranti di Grandi Dimensioni.Hickory Stripe Pant Pantaloni da Lavoro Donna. In the northern part of the district rhodochrosite and rhodonite are essentially contemporaneous, but both thin sections and hand specimens commonly show rhodonite to be veined and partly replaced by rhodochrosite. Hence it is probable that rhodochrosite continued to form after rhodonite. Sam EdelmanFelicia, compact and crystalline, with grains ranging in size from 0.1 mm. to over 6 cm. The color ranges from deep rose to pale pinkish gray and cream. Many specimens are color-BrandDonna 7W83654 Maniche Corte T. Some of the banding is textural, dependent on size of grain and the number and character of minute inclusions. It is evident that much of the rhodochrosite has been deposited in successive layers. About seventy determinations of refractive index of rhodochrosite grains of all colors, grain size and associations were made on specimens from all parts of the southwestern and western portion of the district. The lowestnO noted is 1.778 in the cream-colored band of a sharply-Tricot Track Top Felpa Uomo level of the Emma mine. The highestnO noted is 1.815 in dense pale pink rhodochrosite from the extreme western edge of the district. The great majority of the indices lie between 1.797 and 1.810, with the average at 1.8035. Scarpe da Basket Uomo. In general the light or cream colored rhodochrosite bands are lower in index than the pink or rose carbonates.adidasIniki Runner CLS.Kurz Regular Fit Vestito Donna, though this is generally not true. Clearly there is considerable isomorphic variation in the Butte, rhodochrosite,JockeyBoxer da uomo. Specimen N in Table 1 and Plate 1 is from the 1000-Blocked Sole No Show Socks level of the Emma mine.adidasSet Sportivo. Donna, large, zoned crystal from a coarse-grained, aggregate and has a range in color from deep to medium pink. The determinations of specific gravity were made by J. G. Fairchild using the pycnometer method and by Waldemar T. Schaller using the Berman microbalance. The partial analysis by Fairchild is MnO 55.20, MgO 3.28, CaO 1.68, FeO 0.30, ZnO none, SO30.02, insoluble 0.05, CO239.31 (calculated); total 99.84 per cent. Two bulk analyses of Emma ore are available. Hewett and Pardee 14give the average composition of about 250,000 tons of ore from the Emma mine and indicate the molecular composition of the rhodochrosite free from impurities to be MnCO391 per cent, MgCO37 per cent and CaCO32 per cent.The average percentage composition of 13,961 tons of rhodochrosite ore delivered to the Southern Manganese Corporation in 1918 and 1919 and the variation in monthly average analyses are given below:
Professionali autobloccanti da Corsa Strada Scarpe da Ciclismo su Strada3following Hewett and Pardee, 15the molecular composition of the rhodochrosite free from impurities is approximately as follows:
These analyses and the optical data indicate that Butte rhodochrosite is lower in iron and higher in magnesium than most rhodochrosites. The rhodochrosites of Philipsburg, Montana, specimens U, V, and W, have similar characteristics. OTHER MAGANESE MINERALS AT BUTTE In addition to the abundant rhodonite of the northern part of the district, minor quantities of mangan-ankerite, manganosiderite, 16huebnerite,17helvite18and alabandite19are found. A specimen of pale pink, massive, crystalline mangan-ankerite from the dump of the Bonanza mine was analyzed by J. G. Fairchild: CaO 30.52, MgO 15.92, MnO 4.28, FeO 3.31, ZnO none, SO3none, insoluble none, and CO246.08 (calculated); total 100.11. The observednO ranges between 1.690 and 1.698 and the calculatednO is 1.694 .The specific gravity and molecular proportions are given in Table 2 as specimen q.Greenish wolframite similar to that described as an alteration product of huebnerite by Winchell 20FnhoFnho Outdoor Scarpe da Trekking. The mineral occurs as extremely tiny,Star Seasonal Canvas Low Top Sneaker. Spectrographic tests by K. J.Sciarpa Srinagar Medium Check Dark Blue da Pashmina & Silk, Mn, and W. The identity of the wolframite was proved by x-ray powder pictures taken by W. E. Richmond.ACKNOWLEDGMENTS Seta Premio 4011 da Pashmina & Silk. Waldemar T. Schaller, D. F. Hewett, Charles F. Park, Jr., J. B. Mertie, Michael Fleisher, and Duncan McConnell. The Anaconda Copper Mining Company permitted the examination of the Butte carbonate ores. Persons responsible for the chemical and some of the optical data have been acknowledged throughout the text.HurleyM Phantom Gallows Beachside Boardshorts. E. N. Goddard, W. S. Burbank, C. P. Ross, H. D. Miser, and D. F. Hewett of the U. S.Scarpe da Ginnastica Basse Donna. NOTES * Submitted for publication with the approval of the director of the U. S.Running Sports Jogging Clothes 5pcs Set Compressione Elastica Abbigliamento Casual Ad Asciugatura Rapida. 1 Ford, W. E., Studies in the calcite group:Trans. Conn. Acad. Arts and Sci., 22, 211-248 (1917). 2 Ford, W. E.,op. cit., pp. 219-220. 3 Ford, W. E.,op. cit., p. 218. 4 Krieger, Philip, Notes on an x-ConverseAll Star Hi Canvas-rhodochrosite:Am. Mineral., 15, 23-29 (1930). 5 Ford, W. E.,op. cit., p. 221. 6 Ford, W. E.,op. cit., p. 242. 7 Ford, W. E.,op. cit., pp. 215-228. 8 Brentano, J., and Adamson, J., Precision measurements of x-ray reflexions from crystal powders:Philos. Mag.,ser. 7, 7, 507-517 (1929). 9 Wyckoff, R. W. G., The crystal structure of some carbonates of the calcite group:Am.. Jour. Sci., 4th ser. 50, 317-320; 351-353 (1920). 10 Ewald, P. P., and Herman, C., Structurbericht 1913-1926,Zeits. Krist., 316-317 (1931). Also International Critical Tables, 1, 342 (1926). 11 Bragg, W. L., Atomic Structure of Minerals, Cornell Univ. Press, 114-116 (1937). 12 Wyckoff, R. W. G., The Structure of Crystals, 2nd ed.,Am. Chem. Soc., Mon.New BalanceMen's 430v1 Running Shoe19, 275 (1931). 13 Hewett, D. F., and Pardee, J. T., Manganese in western hydrothermal deposits; Ore deposits of the western states (Lindgren Volume):Am. Inst. Min. Met. Engr., 672-675 (1933). Sales, R. H., Ore deposits at Butte, Montana:Trans. Am. Inst. Min. Met. Eng., 46, 3-109 (1914). Weed, W. H., The geology and ore deposits of the Butte district, Montana:U. S. Geol. Scarpa Unisex, Prof. Paper, 74 (1912). Hart, L. H., The Butte district, Montana; Copper resources of the world:XVI Int. Geol. Congress, 1, 287-305 (1935). Pardee, J. T., Deposits of manganese ore in Montana, Utah, Oregon, and Washington:U. S. Geol. Scarpa Unisex, Bull. 725c, 141-179 (1921). Pardee, J. T., Manganese at Butte, Montana:U. S. Geol. Scarpa Unisex, Bull690e, 111-130 (1918). Perry, Eugene S., The Butte mining district, Montana:XVI Int. Geol. Congress, Guidebook23 (1932). Bard, D. C., and Gidel, M. H., Mineral associations at Butte, Montana: Trans. Am. Inst. Min. Met. Eng., 46, 123-127 (1914). 14 Hewett, D. F., and Pardee, J. T.,op. cit., p. 674. 15 Hewett, D. F., and Pardee, J. T.,op. cit.p. 674. 16 Pardee, J. T.,op. cit.(Bull. 690e), p. 119. 17 Winchell, A. N., Notes on tungsten minerals from Montana:Ec. Geol., 5, 158-165 (1910). 18 Hewett, D. F., Helvite from the Butte district, Montana:Am. Mineral., 22, 803-804 (1937). 19 Hewett, D. F., and Pardee, J. T.,op. cit., p. 674. 20 Winchell, A. N., op. cit., p. 165. |